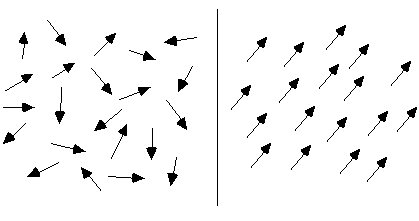
To make the phenomenon of self-organization more concrete, it is useful to look at two basic examples of self-organizing systems. Perhaps the simplest such process that has been extensively studied is magnetization. A piece of potentially magnetic material, such as iron, consists of a multitude of tiny magnets, called "spins" (see Figure 1). Each spin has a particular orientation, corresponding to the direction of its magnetic field. In general, these spins will point in different directions, so that their magnetic fields cancel each other out. This disordered configuration is caused by the random movements of the molecules in the material. The higher the temperature, the stronger these random movements affecting the spins, and the more difficult it will be for any ordered arrangement of spins to maintain or emerge.
 ;
;
Figure 1: two arrangement of spins: disordered (left) and ordered (right)
However, when the temperature decreases, the spins will spontaneously align themselves, so that they all point in the same direction. Instead of cancelling each other, the different magnetic fields now add up, producing a strong overall field. The reason that the spins "prefer" this ordered arrangement is because spins pointing in opposite directions repel each other, like the North poles of two magnets that are brought together. Spins pointing in the same direction, on the other hand, attract each other, like the North pole of one magnet attracts the South pole of another magnet. Magnetization is a clear case of self-organization, which can be used as a paradigm for a whole range of similar phenomena, such as crystallization (where not only the orientations but also the positions of the molecules become evenly arranged).
A somewhat more complex example will illustrate further characteristics of self-organization. In the Bénard phenomenon, a liquid is heated evenly from below, while cooling down evenly at its surface, like the water in an open container that is put on an electric hot-plate. Since warm liquid is lighter than cold liquid, the heated liquid tries to move upwards towards the surface. However, the cool liquid at the surface similarly tries to sink to the bottom. These two opposite movements cannot take place at the same time without some kind of coordination between the two flows of liquid. The liquid tends to self-organize into a pattern of hexagonal cells, or a series of parallel "rolls", with an upward flow on one side of the roll or cell and a downward flow on the other side. This example is similar to the magnetization in the sense that the molecules in the liquid were moving in random directions at first, but end up all moving in a coordinated way: all "hot" molecules moving upwards on one side of the roll, all "cool" molecules downwards on the other side (see Figure 2). The difference is that the resulting pattern is not static but dynamic: the liquid molecules remain in perpetual movement, whereas the magnetic spins are "frozen" in a particular direction.
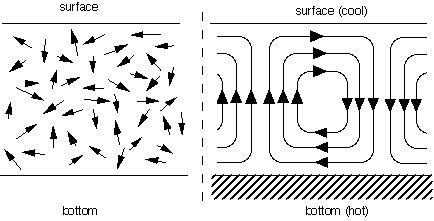
Figure 2: two types of movements of liquid molecules: random (left) and in the form of Bénard rolls (right), caused by a difference of temperature between the bottom and the surface of the container.

